What is Unique Device Identication?
On September 24, 2013, the U.S. Food and Drug Administration (FDA) established a unique device identification (UDI) system to identify and trace medical devices throughout their distribution and use. UDI is expected to improve patient safety and healthcare industry processes greatly by implementing a global system of standards for device identification. This system makes it possible to identify devices and attributes of devices in the market quickly and accurately, ensuring safety and effectiveness. In the case of adverse events such as a product recalls, devices can be rapidly and precisely identified to take corrective action, minimizing risk to patients and consumers.
As part of the FDA UDI mandate, medical devices must bear a unique device identifier (UDI) code in human-readable (text) and machine-readable (barcode, RFID) format on labels or packag- ing (the UDI code may be provided in either or both human- or machine-readable format for direct marks).
What Is the Required Format of a UDI Mark?
Unlike UDI codes on labels and packaging, when an UDI is directly marked on a device, the UDI code may be provided in either or both of the following formats:
- Human-readable: Easily-legible, plain-text format.
- Machine-readable: Able to be interpreted by automatic identi cation and data capture (AIDC) technology, such as barcode readers, machine vision systems, RFID equipment, or any other technology that will provide the UDI code to databases on demand.
These format options for UDI direct marks are provided to accommodate devices that have limited surface area or other constraints regarding how and where marks can be applied.
Which Data Carriers Should Be Used for Machine-Readable Marks?
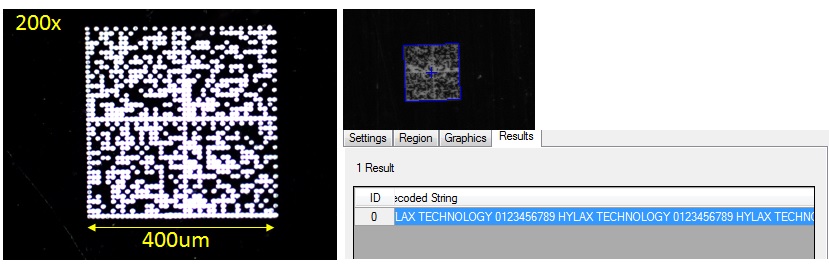
Machine-readable UDI codes are encoded into what are called “data carriers” (the machine-readable media that carries the UDI data; for example, barcodes, RFID chips, or other media). The FDA does not specify which data carrier must be used by the manufacturer for UDI compliance, but considerations should be made regarding issuing agency specifications for a given manufacturer or device, the types of data carriers the manufacturer’s customers are able to read, and the types of data carriers that can be easily marked and read on medical devices.
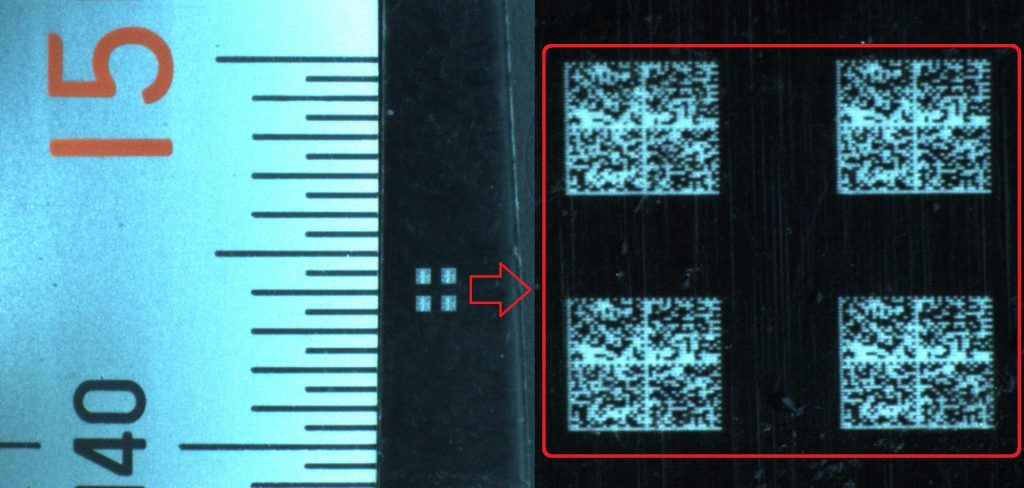
2D symbols such as Data Matrix are the most common data carriers used for direct part marking because of their small size, high data capacity, and built-in error correction. Data Matrix symbols consist of an arrangement of small dots or squares used
to encode data. Compact 2D symbols like these are more easily and accurately marked on devices than lines of text or one dimensional codes like linear barcodes. 2D symbols can also be marked by a variety of methods (laser, dot peen, or electrochemical etch).
The data-carrying capacity of Data Matrix symbols makes it easy to accommodate large data volumes in small spaces, like the surface areas of some medical devices.
Data Matrix symbols are extremely reliable and can be read in any orientation, at low or high contrast, and even when errors are present. The symbology standard for Data Matrix includes error detection and correction algorithms to ensure reliable reading despite parts of the symbol being obscured or missing. This is a helpful feature in environments where symbols may be obscured by grease, dirt, paint and chemical coatings, and when the symbology is applied to metal and other reflective surfaces. As a result of error correction, Data Matrix symbols with damage, distortion, or minor defects can still be decoded accurately, even if more than twenty percent of the symbol is obscured.
Direct marking is an important component of the FDA Unique Device Identication (UDI) regulation. The purpose of UDI is to establish a standard method for identifying and tracing medical devices throughout their lifecycles – from production, to distribution, to use. For medical devices that are reused many times during their lifecycles, a UDI must be able to withstand any amount of handling or reprocessing. The most reliable way to ensure a UDI lasts the lifecycle of a multi-use device is by affixing a permanent mark to the device itself, rather than a temporary package or label. Direct part marking (DPM) is not a new concept in industrial manufacturing, but is relatively new territory for medical device identification.
Laser marking is an effective and suitable way to implement this direct marking. The advantages includes:
- Permanent mark able to withstand daily usage and cleaning processes
- Able to achieve high contrast with special methods
- Able to mark without surface deformation to achieve cleanliness quality comparable to unmarked portion
- Achieve extremely small marks below 500 um size for a data matrix

Hylax has many years of experience in assisting customers in this requirement. We have designed laser markers incorporated with inline vision to register the exact mark location in a loosely placed part and subsequently to verify the mark quality.